The pharmaceutical industry is a key part of the global economy and human health, based on the complex world of bulk drugs. Three connected components are fundamental in this sector: Active Pharmaceutical Ingredients (APIs), Key Starting Materials (KSMs), and drug intermediates. Together, these elements form the backbone of medicine manufacturing, ensuring billions of patients around the world receive effective and safe treatments.
Without them, modern healthcare would be unrecognizable. While consumers usually see the final products like tablets, capsules, syrups, or injections at pharmacies, the real work begins earlier in chemical plants and biopharmaceutical facilities where APIs, intermediates, and KSMs are created.
Understanding APIs, Intermediates, and KSMs
APIs are the active substances that provide a medicine’s therapeutic effect. They are part of a drug formulation responsible for the desired benefit, such as paracetamol in a pain relief tablet or atorvastatin in a cholesterol-lowering drug. Drug intermediates are the chemical compounds created during the stages of synthesis. Although they are not active medicines yet, they are vital building blocks that eventually develop into the final API through further processing.
KSMs are the raw chemical materials that start the synthesis of intermediates and APIs. Each step connects seamlessly, starting with KSMs, moving into intermediates, and ending with APIs, which are then made into finished dosage forms for patients.
Shifting Focus on Local Manufacturing
In recent years, people have focused on the manufacturing of APIs, KSMs, and drug intermediates due to vulnerabilities in supply chains, increasing healthcare demands, and policy changes favoring local production. During the COVID-19 pandemic, the world saw how disruptions in the supply of APIs and intermediates could cripple healthcare systems.
This prompted many governments and pharmaceutical companies to rethink their sourcing strategies, limit reliance on a few suppliers, and enhance local production capabilities. For entrepreneurs and new businesses, this change has presented numerous opportunities, from manufacturing in-demand bulk drugs to developing niche products in specialized therapeutic areas.
Market Growth and Demand Drivers
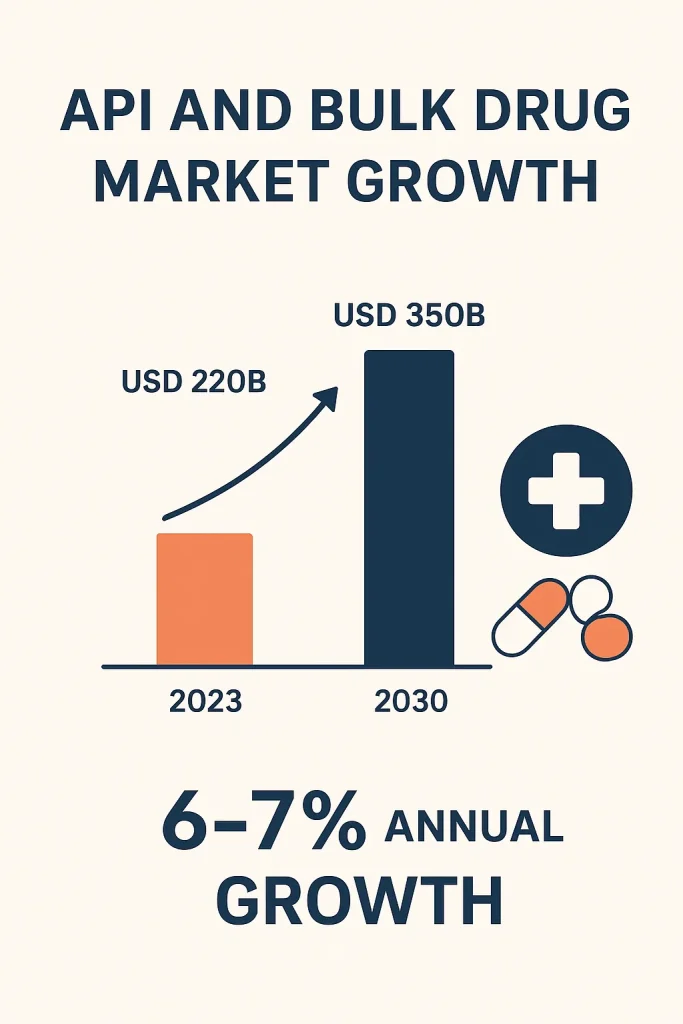
The API and bulk drug market have shown strong growth. Industry estimates suggest the global API market exceeded USD 220 billion in 2023 and is expected to grow steadily at an annual rate of about six to seven percent from 2024 to 2030. This growth results from several factors. Chronic illnesses, including cardiovascular disease, diabetes, and cancer, create an ongoing demand for medicines.
An aging population adds to this need, as older adults generally require more healthcare. Additionally, the expiration of patents on many popular drugs has led to an increase in generics, significantly raising the consumption of APIs and intermediates.
Detailed project report on APIs-KSMs-Drug Intermediates Bulk Drug
KSMs and Global Supply Dependence
KSMs and intermediates are central to this system. Historically, China has been the leading supplier of these materials, with many countries depending almost entirely on Chinese manufacturers for essential raw ingredients. However, worries about overreliance, environmental compliance, and trade restrictions have led countries like India, the United States, and several European nations to push for local production of KSMs and intermediates.
For example, the Indian government has created a Production Linked Incentive (PLI) scheme to encourage the manufacturing of bulk drugs and KSMs, decreasing dependence on imports while also boosting domestic abilities. This concern goes beyond economics; ensuring healthcare self-reliance is crucial for maintaining the production of necessary medicines during crises.
Opportunities for Entrepreneurs
For entrepreneurs, the connected chain of KSMs, intermediates, and APIs offers many entry points. Some may focus on producing KSMs, which are often imported in large amounts and are essential to the bulk drug industry. Others might see an opportunity in specific intermediates for high-demand therapeutic areas like oncology, antibiotics, or antiviral treatments.
Still, others may aim for full-scale API manufacturing, which, although requiring significant capital and heavy regulation, offers major long-term gains. Beyond mainstream drugs, niche areas like rare disease treatments, biopharmaceutical APIs, and customized intermediates for research and development are becoming more profitable.
For further information, please consult our books.
The Manufacturing Process
The manufacturing of APIs and intermediates is intricate and demands precise coordination of chemistry, biotechnology, and quality assurance. It starts with sourcing KSMs, which must meet strict quality requirements to prevent impurities from affecting later reactions. These raw materials go through multi-step synthesis to create intermediates, with processes that may include organic synthesis, fermentation, or enzymatic changes.
Each reaction occurs under tightly controlled conditions of temperature, pressure, and pH to produce the desired compound. Once intermediates are formed, further reactions and refinements lead to the production of APIs. These APIs are then purified using advanced techniques like crystallization, chromatography, or filtration, ensuring they meet quality standards for purity and effectiveness. Finally, APIs undergo rigorous testing for safety, stability, and efficacy before being packaged and supplied to pharmaceutical companies that turn them into final drug products.
Regulatory Landscape
The regulatory landscape in this industry is among the strictest globally due to the high stakes of human health. Manufacturers of APIs, KSMs, and intermediates must follow Good Manufacturing Practices (GMP) as specified by authorities like the US Food and Drug Administration (USFDA), the European Medicines Agency (EMA), and the World Health Organization (WHO).
They also need to comply with environmental rules, including proper waste treatment and disposal, since bulk drug manufacturing can create hazardous by-products. Another crucial element is the submission of Drug Master Files (DMFs), which provide regulators with detailed information on the manufacturing processes, controls, and facilities used in API production. Adherence to intellectual property laws is also essential, as making patented APIs without permission can lead to legal issues.
Related articles:- The Indian Pharmaceuticals Sector
Emerging Trends in the Industry
New trends in the API and bulk drug industry are changing how businesses function. APIs derived from biotechnology, known as bAPIs, are gaining popularity as therapies increasingly shift towards biologics, including monoclonal antibodies, peptides, and recombinant proteins. Continuous manufacturing methods, which streamline traditional batch processes into flow systems, are enhancing efficiency, cutting costs, and improving scalability.
The focus on sustainability and green chemistry is also growing, with companies adopting eco-friendly solvents, energy-efficient processes, and waste reduction strategies. Incorporating digital technologies such as artificial intelligence, machine learning, and big data analytics is also helping optimize processes, enable predictive maintenance, and ensure quality. These improvements not only enhance competitiveness but also align companies with global regulatory and environmental expectations.
Challenges Facing the Sector
Despite the many opportunities, challenges persist in the industry. High barriers to entry, due to strict regulations and the need for considerable capital investment, make it hard for smaller players to establish themselves. Relying on imported raw materials, particularly specialized KSMs, can expose businesses to supply chain disruptions. The market is highly competitive, especially for commoditized APIs, where large-scale producers from China and India dominate.
Furthermore, some advanced APIs require very sophisticated technology, such as bioreactors or state-of-the-art fermentation units, further complicating and increasing the cost of production. However, these challenges also serve as filters, ensuring that only serious, quality-focused companies thrive in the long term.
Long-Term Prospects
For entrepreneurs looking to enter this market, long-term prospects remain bright. The demand for APIs, intermediates, and KSMs is closely linked to global healthcare needs, which are continuously growing. This ensures a stable and resilient demand, less affected by economic fluctuations than many other sectors. Businesses can start small, focusing on niche intermediates or contract manufacturing before moving into complete API production. Export opportunities are also significant, as regulatory-compliant facilities in developing nations can supply developed markets in Europe, North America, and elsewhere. By focusing on quality, compliance, and sustainability, new entrants can build strong reputations and secure lasting partnerships with global pharmaceutical companies.
Role of Consultancy Services
Organizations like Niir Project Consultancy Services (NPCS) are essential in helping new businesses navigate the technical, regulatory, and financial challenges of this sector. NPCS creates detailed techno-economic feasibility reports that cover everything from sourcing raw materials and process design to plant layouts, cost structures, and market forecasts.
For entrepreneurs unfamiliar with the complexities of chemical engineering and pharmaceutical regulations, this expertise is crucial for assessing risks, anticipating problems, and spotting profitable opportunities. With proper guidance, even first-time entrepreneurs can build successful businesses in this specialized industry.
The Bigger Picture
The importance of APIs, KSMs, and drug intermediates goes well beyond business. They form an invisible backbone of global healthcare, making sure treatments for everything from minor fevers to life-threatening cancers are accessible and affordable. Their production involves a combination of science, technology, and entrepreneurship, with each step contributing to the creation of life-saving medicines.
As the world confronts new health challenges, including pandemics, rising diseases, and increasing healthcare costs, the role of bulk drug manufacturing will grow more essential. For governments, businesses, and entrepreneurs, investing in this sector offers not just a commercial chance but a contribution to human well-being.
Conclusion
In conclusion, the interconnected chain of APIs, KSMs, and drug intermediates forms the backbone of the pharmaceutical industry. Their production requires a careful balance of chemistry, compliance, innovation, and market dynamics. Despite challenges like regulatory obstacles, supply chain risks, and price competition, the growth opportunities are vast and expanding.
Continuous demand for medicines, the global drive for self-sufficiency, and the advent of new technologies all point to a promising future for this industry. For entrepreneurs and businesses ready to focus on quality and innovation, the bulk drug sector offers a route to both commercial success and a significant role in shaping healthcare’s future.
Discover best business ideas for yourself using our startup selector tools
Frequently Asked Questions
Q1. What are KSMs in pharmaceuticals?
KSMs (Key Starting Materials) are raw compounds that serve as building blocks for drug intermediates and APIs.
Q2. Why are APIs and KSMs important?
They form the foundation of pharmaceutical manufacturing, ensuring a consistent supply of essential medicines.
Q3. What is the market outlook for APIs and intermediates?
Global demand continues to rise due to healthcare expansion, chronic disease treatment, and export opportunities.
Q4. What trends are shaping the API and KSM industry?
Eco-friendly production, backward integration, and reliance on domestic supply chains are emerging trends.
Q5. How does NPCS support startups in this sector?
NPCS helps entrepreneurs with market survey reports, feasibility studies, and detailed process know-how.






