The healthcare market in the world is at an all time boom and medical device manufacturing is a lucrative market to venture in. The necessity to supply critically needed medical supplies is growing steadily as the population of aged citizens, as well as cases of chronic diseases, continues to grow. Disposable intravenous sets provide a business opportunity among all the necessities of the healthcare product.
The medical sector is therefore a good environment that an entrepreneur and a startups would want to venture into the medical industry as it will have prolonged demand, and the long run prospects of expansion are healthy!
Learning about Disposable IV Sets: The Lifeblood of 21 st century healthcare.
Whenever you walk into a hospital corridor, you will always find the incessant use of IV dripps, which provide the patient with fluids and required drugs. It may be a straightforward procedure, however, it should be noted that the medical instrument behind it, the disposable IV set, is a complex one, which entrepreneurs cannot even discover, but can easily cure millions of treatments daily, day and night, virtually, a sterile, single-use, complete medical device.
The fascinating thing about a disposable IV set as a business is that, everybody requires it. The use of IV set is an essential tool in any healthcare institution. Disposable IV sets are essential whether in a large city hospital that carries out complex surgeries or in a small rural medical facility where patients are taken in due to cases of dehydration.
Report on Disposable IV Set Detail project report.
Market Forecast: An Increasing Sales Potential That Can hardly pass unnoticed.
The figures are a powerful narrative to anyone who would want to pursue this business opportunity. The global market in disposable IV set has been constantly on the rise in recent years, and today the estimates by the industry analysts show that it is going to rise to approximately eight billion dollars by the next decade in comparison to the current four and a half billion dollars. That is, this is healthy annual growth rate of nearly seven percent- a growth rate of constant expansion that not only is a growth that is worth of confidence but also allows the businesses to strategize to achieve success in the long run.
The wider market of infusion therapy is also undergoing the same growth with an indication that it is likely to grow between twelve and a half billion dollars to over eighteen billion dollars within the same period. Such a general increase is an indication that the drivers of demand are solid and long term and not a reality of short term market fluctuations.
To entrepreneurs who may be questioning the viability in the long run, it should be noted that there are more than two hundred and thirty million primary IV sets used in around two hundred and fifty healthcare facilities around the world every year. That is not going to be such a figure that will reduce in the near future. This need will only keep rising as the healthcare systems continue to grow across the world and medical treatments get more advanced.
Market Outlook:-

There are many strong trends that are converging to produce unbelievable opportunities in disposable IV set market. The most profound long-term force source is the aging population that is already entailing the increased life span and, respectively, the increased medical care, – most of it, quite naturally, will be IV therapy.
This is not a problem of such countries as the US in which the average age is older; the early emerging countries are also feeling a demographic tailwind which is beginning to pan on the enhancement of their health care system and this translates to a very big global market of disposable IV sets.
The US and developing countries are increasing the chronic disease, and this calls demand of medical care and treatments that require the IV administration. The chronic condition tends to create demand which is more stable and predictable- contrary to an acute one such as broken bone where the demand would be short time with medical consumables like IV sets.
New potential customers, who require a supplier and a consistent/professional supplier of IV sets, are ambulatory surgery centers, special treatment clinics and home health car.
The entire health care infection control is increasing very rapidly also and hence exact demand/ desire of always-good quality disposable IV sets is increasing rapidly.
To get more ideas go to our handbook
Manufacturing Process: Transforming the Raw Materials into Life-Saving Devices.
It is important that the entrepreneurs get to know the manufacturing process in the event they want to seize on this business opportunity. Although it is advanced, it is not too complicated as long as companies are well-capitalized with appropriate technical skills and regulatory oversight.
Beginning With the Right Materials.
The initial phase of any quality IV deal with the first important step of material choice. To begin with, medical grade plastics must pass the biocompatibility criteria with very rigorous tests even with extreme sterilizing agents.
Mostly, the materials that will be used will consist of special PVC on the flexible part the tubing and Polypropylene on the rigid components of the product, i.e., the drip chambers.
Accurate Production Operations.
Such parts are not mere plastic components! Every component should be of a specific dimension precision, and similar performing features with millions of other units.
The extrusion process consists of melting PVC pellets and subsequently forming the same to extruded tubes of continuous lengths of tubing, as long as you can sustain internal and external diameter as specified. Extrusion, the plastic undergoes heat and has precision advantages in regard to feedback of the dimensions which are being extruded.
Lastly, after the manufactures have made the specified components of the IV set, there is a form of assembly of the components that also needs to be done alongside quality checks.
Probably the most important point is the process of assembly, and it should take place in the cleanrooms that have to be made under strictly controlled conditions. Each step will be handled involving great attention to the maintenance of sterility and quality control. The employees are guided by elaborate manuals to attach parts, fit filters, secure clamps and ensure every set finished is up to specs.
All manufacturing stages are incorporated with quality. Some leak testing, the flow rate checking, dimensional checking, and visual checking are done prior to products being transported to the next stage. This is an inclusive strategy necessary in fulfilling the regulations and customers satisfaction.
Sterilization and Packaging
The final manufacturing steps involve sterilization using validated processes that eliminate all microorganisms while maintaining product integrity. Whether using ethylene oxide gas or gamma radiation, manufacturers must carefully control sterilization parameters and verify effectiveness through rigorous testing protocols.
Quality Standards and Regulatory Compliance
Quality isn’t optional in medical device manufacturing – it’s the foundation everything builds upon. In the United States, IV sets fall under Class II medical device regulations, requiring manufacturers to comply with comprehensive quality system requirements and often obtain premarket clearance before selling their products.
International quality standards for this industry include ISO certification for medical device quality management systems, along with specific standards for infusion equipment. To comply, manufacturers must establish quality management systems that encompass design controls, risk management, supplier controls, production controls, as well as post-market surveillance.
In addition to meeting these requirements, quality control testing plays a critical role in ensuring product reliability and safety. For example, leak testing verifies component integrity, while flow rate testing confirms proper fluid delivery. Furthermore, material compatibility testing validates biocompatibility, and finally, sterility assurance level verification guarantees that each product meets stringent safety standards before reaching the market..
Related articles:- How to Start a Surgical Adhesive Plaster Manufacturing Business
Market Applications: Understanding Your Customers’ Needs
Successful IV set manufacturers understand that different customer segments have distinct needs and purchasing patterns. Hospital settings represent the largest market segment, accounting for approximately forty-five percent of total demand due to high surgical volumes and comprehensive healthcare services.
Ambulatory surgical centers represent one of the fastest-growing segments, driven by healthcare system efforts to reduce costs and improve efficiency. These facilities often prefer standardized product packages that simplify inventory management and reduce waste.
The home healthcare market presents unique opportunities. Products used in home settings must be more user-friendly than those used by trained hospital staff while maintaining the same safety standards. Specialty clinics focusing on treatments like chemotherapy, dialysis, or chronic pain management often require IV sets with particular features, typically offering higher margins and more stable customer relationships.
Related articles:-Acrylonitrile, the essential chemical driving modern industries
Strategic Market Entry: Building Your Path to Success
Entering the disposable IV set market requires careful strategic planning, addressing manufacturing capabilities, regulatory compliance, and customer development simultaneously. Many successful startups begin by partnering with established contract manufacturers while developing their own capabilities, allowing faster market entry with lower initial capital requirements.
Regulatory strategy deserves special attention because compliance issues can derail even well-funded ventures. Smart entrepreneurs engage regulatory consultants early and build compliance costs into their business models from the beginning.
Customer relationship development takes time and requires dedicated effort. Healthcare customers move cautiously when evaluating new suppliers because product failures can directly impact patient care. Building trust requires consistent demonstration of quality, reliability, and responsive customer service.
Professional Support and Future Prospects
NIIR Project Consultancy Services (NPCS) has extensive experience helping entrepreneurs navigate the challenges of establishing manufacturing ventures in the disposable IV set industry. NPCS prepares comprehensive Market Survey cum Detailed Techno Economic Feasibility Reports that provide a thorough analysis of market opportunities, manufacturing processes, raw material requirements, plant layout optimization, and detailed financial projections. Their industrial project development expertise helps entrepreneurs assess the feasibility of setting up new industries or businesses, providing the foundation for informed investment decisions and successful project implementation.
The disposable IV set market continues evolving with technological advancements. Smart IV sets incorporating sensors for flow monitoring represent emerging opportunities combining traditional manufacturing with modern electronics. Safety features continue evolving as healthcare systems seek products that reduce medication errors and improve patient outcomes.
Conclusion:-
The disposable IV set manufacturing sector offers compelling opportunities for entrepreneurs who approach the business with proper preparation, adequate capitalization, and commitment to quality excellence. The fundamentals are strong: consistent demand growth driven by demographic trends, clear regulatory pathways for market entry, and diverse customer segments that value reliable suppliers.
Success requires serious commitment to quality systems, regulatory compliance, and customer relationship development. But for entrepreneurs willing to make these investments, the disposable IV set market provides a stable foundation for building substantial, profitable businesses that contribute meaningfully to global healthcare delivery.
The healthcare industry will always need reliable partners who can consistently deliver quality medical supplies. For entrepreneurs ready to meet this challenge, the disposable IV set market represents an opportunity to build businesses that are not only financially rewarding but also contribute directly to improving patient care outcomes worldwide.
Find Best business iseas for yourself using our startup selector tools
Frequently Asked Questions
1. What is a disposable IV set?
Healthcare providers use a disposable IV (intravenous) set as a single-use medical device to deliver fluids, nutrients, or medications directly into a patient’s vein under controlled flow conditions.
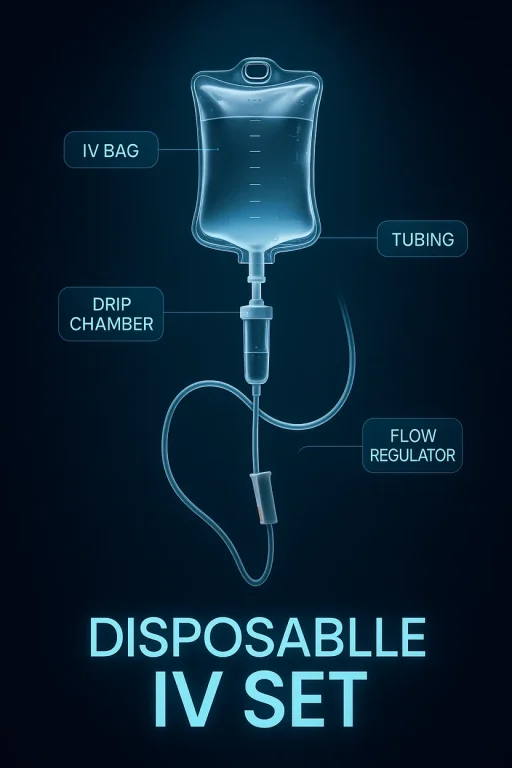
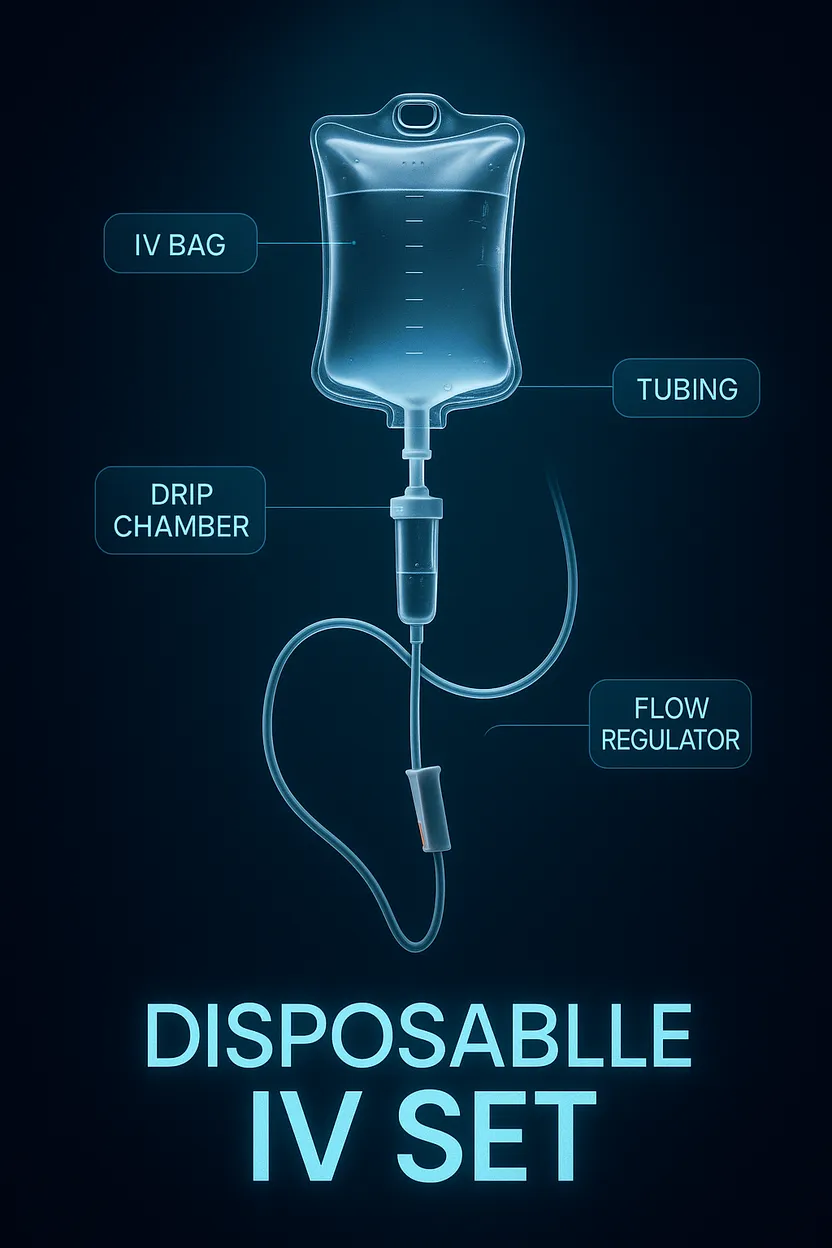
2. What components are included in a disposable IV set?
A standard set typically includes:
- Drip chamber
- Spike (for inserting into the IV fluid container)
- Tubing
- Flow regulator (roller clamp)
- Injection port or Y-site
- Needle or catheter adapter
3. Is the IV set sterile?
Yes, disposable IV sets are sterile and are individually packed to prevent contamination. They must be discarded after one use.
4. How is a disposable IV set used?
The spike is inserted into an IV fluid container, the drip chamber is partially filled, and the tubing is primed to remove air. It is then connected to the patient’s venous access point for fluid administration.
5. Can a disposable IV set be reused?
No. For patient safety and to prevent infection, IV sets are designed for single use only and should be disposed of according to biomedical waste protocols.