2, 3-dihydroxysuccinic Acid (Synthetic tartaric Acid) is A white, crystalline chemical compound that can be prepared and derived either from the synthetic route or natural sources. Tartaric acid has natural sources in grapes, tamarinds, and bananas, among other fruits.
Tartaric acid can also be prepared industrially from maleic anhydride or hydrogen peroxide. It has three stereoisomeric forms: L(+), D(−), and meso tartaric acid.
Racemic forms DL-Tartaric acid L-(+)-Tartaric acid is known as the Wohlgemuth acid, and it is widely used in the food industry due to its antimicrobial characteristics, whereas D-(-) – Tartaric acid occurs naturally in wines.
Business Plan: Tartaric Acid Production Business
Some Uses:
Many industries, including agriculture, food, cosmetics, and detergents, use synthetic tartaric acid, often as a food preservative or antioxidant. It is also an ingredient of some medicines and toothpastes.
Manufacturers produce this type of acid by treating the ammonium salt of 2‑methyl butanoic acid with sulfur trioxide or sulfur dioxide at elevated temperatures. The reaction produces sodium sulfate along with other byproducts and yields sufficient amounts of artificial tartaric acid. As it is an organic compound, it may be present in animal feed and drinking water.
Visit this Page for More Information: Start a Business in Chemical Industry Projects
Synthetic tartaric acid can be obtained from natural sources like grapes, tamarinds, and date palm fruit pits. It has a low toxicity level when compared to other acids, such as citric acid or acetic acid. It does not dissolve easily in water; however, it dissolves well in alcohols, ethers, and glycols at room temperature.
In addition, it is soluble in chloroform and benzene solvents. This chemical has poor color stability and hence should be stored in light-proof containers under refrigerated conditions. It exhibits excellent fire-retardant properties that make it suitable for use as a flame retardant for textiles, rubber, and plastics.
In addition, it prevents fats from becoming rancid while preserving their flavor. Furthermore, synthetic tartaric acid acts as an emulsifier that facilitates mixing two immiscible liquids together.
Read more similar articles: Chemical Industry
General overview of production process of synthetic tartaric acid:
About 30% of tartaric acid comes from the maleic anhydride and hydrogen peroxide. The process hydrates maleic anhydride and hydrogen peroxide to produce synthetic tartaric acid and was dried with a vacuum evaporation system.
The process generally takes about 24 hours but sometimes some manufacturers shorten the process time to 3–5 hours. Lastly, they pack synthetic tartaric acid.
Why should you invest in this Business Idea?
There is a strong global demand for both organic and synthetic tartaric acid. Its antibacterial, anti-caries, and anti-plaque properties drive significant market growth, as manufacturers widely use it in toothpaste, mouthwashes, lozenges, chewing gums, dental floss, and other oral care products.
The demand continues to rise at a rapid pace, yet India currently has no domestic manufacturer of this compound. This gap makes the country an attractive destination for establishing a new tartaric acid manufacturing industry.
Related Feasibility Study Reports: Chemicals (Organic, Inorganic, Industrial) Projects
To add on, there are no major manufacturers of Synthetic Tartaric Acid in the Asia Pacific region. You can take advantage of this situation by setting up a new business in India. Because you are going to manufacture your own product from scratch, you will be able to avoid several middlemen and distributors who take a big cut out of profits as commission/sales charges. By cutting out these middlemen, you will be able to make more profit with the same amount invested in your project.
Read our Books Here: Chemical Technology (Organic, Inorganic, and Industrial), Fine Chemicals
Market Outlook
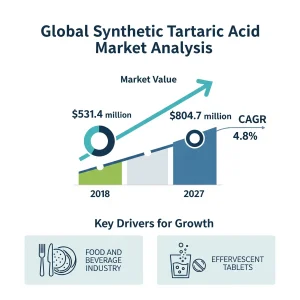
The global synthetic tartaric acid market was valued at US$531.4 Mn in 2018 and is anticipated to expand at a CAGR of 4.8% from 2019 to 2027. The estimated value is US$804.7 Mn by the end of the forecast period. This expansion is projected to bring in an additional $273.3 million between 2019 and 2027.
The global food & beverages industry is growing at a fast pace, and it is expected to be continued over the forecast period. It has been growing because of a population increase
changing eating habits, rising per capita spending, technological developments in food and beverage manufacturing, and an increase in food product shelf life, among other things.
Furthermore, rising demand for this compound as an excipient in effervescent tablets, powders, and granulate formulations will propel the market forward. In addition, governments and regulatory agencies in developed countries are working to shorten the excipient licensing procedure, enabling faster industry growth. Over the projection period, the increased use of chemical admixtures will further drive demand for tartaric acid as a retardant.
Find Detailed Project Report on Project profile page
Frequently Asked Questions
Q1. What is synthetic tartaric acid?
Synthetic tartaric acid, also known as 2,3-dihydroxysuccinic acid, is a white crystalline compound that can be produced chemically or extracted from natural sources.
Q2. Where is tartaric acid found naturally?
It occurs naturally in grapes, tamarinds, bananas, and several other citrus fruits.
Q3. How is synthetic tartaric acid produced chemically?
It can be manufactured using maleic anhydride or hydrogen peroxide, or by treating the ammonium salt of 2‑methyl butanoic acid with sulfur trioxide or sulfur dioxide at high temperatures.
Q4. What are the stereoisomers of tartaric acid?
Tartaric acid exists in three stereoisomeric forms: L (+), D (−), and meso tartaric acid.
Q5. What is DL-tartaric acid?
The racemic form, DL-tartaric acid, is a 50/50 mixture of L (+) and D (−) isomers.






