Sugarcane is a surprising contender in our search for sustainable energy sources. It’s also kind to the planet. This tall grass is capable of providing a cleaner fuel alternative to gasoline, ethanol. From the sugary juice obtained from sugarcane stalks, to a biofuel capable of powering engines is a testimony to both human ingenuity as well as nature’s bounty. This technology, which is particularly popular in Brazil, turns a simple agricultural crop into an important tool for our fight against climate changes and energy independence.
Explore this fascinating process to see how manufacturers transform sugarcane into renewable energy.

How to Get Ethanol: Detailed Overview
A series well-defined processes help Sugarcane to transform into ethanol, with each step playing an important role. So, thinking of Setting up a Profitable Ethanol Manufacturing Plant?
Harvesting and extracting sweeteners from the field to the mill
People harvest mature sugarcane stalks by hand or with machines. They move fast because the sucrose starts breaking down right after cutting. Workers rush the stalks to the mill, aiming to get them there within a day. If they take too long, the sugar content drops, and the value goes down. Speed really matters in this business.
The mill cleans the sugarcane thoroughly to remove dirt, leaves and other debris. It is then processed by powerful machinery which first slashes and chops the stalks. This increases the surface area to extract juice efficiently. The cane is then shredded and passed through heavy rollers which exert enormous pressure to squeeze out the juice. In order to maximize the sugar recovery, it is common practice to spray hot water over the crushed cane, now called bagasse, between roller sets. This helps extract any remaining sugar. Raw sugarcane juice, a brownish green liquid, contains about 10-15% sucrose along with minerals and other organic compounds.
Read More: How to Start Ethanol Production from Sugarcane Business in India
The Magic of Fermentation – Yeast’s role
Here’s how it goes. First, you squeeze the sugarcane juice and get it ready for fermentation. You heat the juice to kill off unwanted bacteria. You filter out the junk and adjust the pH so the yeast can do its thing. Then, you pour the clean, prepped juice into big fermenting tanks.
At this point, you add a special yeast—Saccharomyces cerevisiae, the same one people use for beer and bread. The yeast gets right to work, munching on the sugars and turning them into ethanol. It’s pretty straightforward: prep, pour, add yeast, and let the fermentation roll.These microorganisms are able to metabolize the sugars. The microorganisms consume sucrose and other simple sugars in the juice, and through biochemical reactions produce ethanol and carbon dioxide. This process is represented by the following simplified chemical equation:
C12H22O11 (Sucrose)+H2O (Water)-2C6H12O6 (Glucose/Fructose) C6H12O6 (Glucose/Fructose)Yeast“>2C2H5OH (Ethanol)+2CO2 (Carbon Dioxide)
Fermentation usually takes between 6 and 12 hours. Someone needs to keep an eye on the tanks the whole time because yeast heats things up as it works. If the temperature gets out of control, the whole batch can go sideways.
When fermentation finishes, you get what people call “wine” or “fermented mash.” It ends up with about 7 to 12% alcohol, some water, dead yeast cells, and whatever other leftovers are floating around. It’s not pretty, but it does what it’s supposed to do.
Read Our Book: Handbook on Biofuel, Ethanol and Bioenergy Based Products
Purification through Distillation: Separating Water and Ethanol
It is not pure enough to use as fuel. distillation is used to concentrate the ‘wine’. This technique uses the difference in boiling points between ethanol (78.3C, or 173F), and water (100C, or 212F).
The fermented mash will be pumped into a column. This is a tall tower with trays or plates. The ethanol, which has a lower boiling point than water, is the first to vaporize and rise up the column as the liquid heats. The vapor cools as it ascends and condenses into liquid. It then re-vaporizes onto the different trays. Each cycle of condensation and revalorization purifies ethanol vapor further, increasing its concentration. The liquid stillage or vinasse is the result of water and other components that are less volatile.
This initial distillation usually yields hydrous-ethanol, which contains 95-96% pure alcohol and 4-5% of water. This form of ethanol can be used as fuel for flexible-fuel vehicle (FFVs).
Read More: Molasses-Based Ethanol Production: A Profitable Biofuel Business
Reaching Fuel Grade: The Dehydration stage
To blend ethanol with gasoline in vehicles, the water content must be less than 0.5%. (99.5% ethanol or more). This level of purity is only possible with an extra step called Dehydration. This cannot be achieved by traditional distillation because ethanol and the water mixture form azeotrope. This is a mixture which boils at constant temperature making it difficult to separate further.
Several dehydration methods are employed. molecular Sieve Dehydration is a common and effective technique. This process involves passing the hydrous ethanol through a bed made of specialized materials such as zeolite bead, which has microscopic holes of an exact size. The pores of these beads are large enough for ethanol molecules to pass, but small enough to trap the water molecules and effectively separate any remaining water. The result is anhydrous ethanol of high purity that meets fuel grade specifications and can be blended with gasoline.
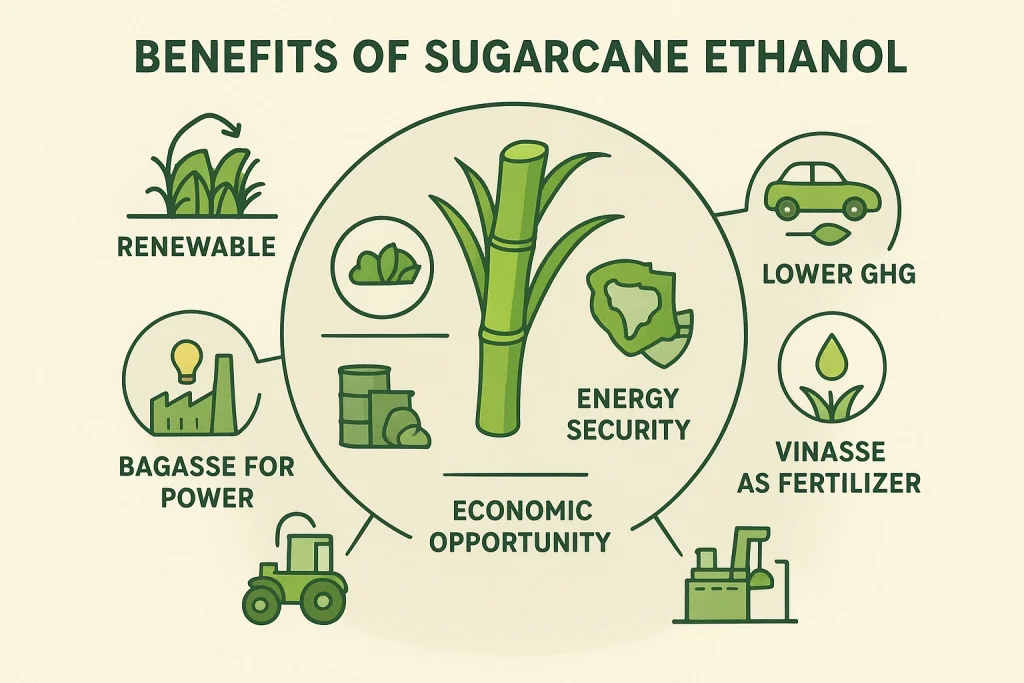
Sugarcane Ethanol: Benefits and Considerations
It is a renewable fuel that offers many advantages.
- Renewable: Sugarcane can be harvested and grown repeatedly unlike fossil fuels. This guarantees a constant supply of raw materials for ethanol production.
- Lower Greenhouse Gases: Ethanol is a fuel that emits fewer greenhouse gasses, especially carbon dioxide, than gasoline. The sugarcane plants also absorb CO2 during their growth. This creates a partial carbon-cycle and reduces the carbon footprint.
- Energy security: In countries where sugarcane is grown, the production of ethanol reduces a country’s dependence on imported oil. This increases their energy independence.
- Economic Opportunity: Sugarcane ethanol creates jobs for agriculture, processing plants and transportation sectors. This industry contributes to economic development in rural areas, especially.
- Waste Valorization Primary byproducts are not wasted. Bagasse is the fibrous residue that remains after juice extraction. It’s commonly used to generate heat and power for the mill. This makes the process self-sufficient in energy and allows for the sale surplus electricity. Vinasse is a liquid waste that can be used to fertilize sugarcane fields. It’s rich in nutrients, and it closes the loop by reducing waste.
Read Niir Project Report Focused On Alcohol And Ethanol Production
It’s important to recognize the challenges and concerns associated with sugarcane-ethanol production.
- Land use: Large scale sugarcane farming can require large land areas. This could lead to deforestation or competition with food production. To mitigate these impacts, it is crucial to use sustainable land management practices.
- Water Use: Sugarcane farming can be water intensive in certain regions. Water management and efficient irrigation strategies are essential to ensure a sustainable use of water.
- Biodiversity impacts: The expansion of sugarcane plantations in natural habitats may negatively impact biodiversity. To minimize these risks, it is essential to adhere to environmental regulations and plan carefully.
Want To Know About Which Business Idea Would Be Better For You?
Go Through Our Startup Selector Tool
Conclusion: A sweet path to a sustainable future
It is remarkable how agriculture can help create a sustainable energy future. We can use the combined power of biology, engineering and science to convert the energy captured by sugarcane into a liquid fuel that burns cleaner. Sugarcane ethanol’s integrated production process, which uses by-products to increase energy efficiency and reduce waste further enhances its sustainability. Sugarcane ethanol is a well-established and vital renewable energy source. It offers a sweet solution to a greener future and plays a key role in global transition towards an energy-secure and sustainable world.
Sugarcane Juice Ethanol Production: FAQs (Frequently Asked Questions)
1. What is ethanol exactly?
It’s just alcohol—yep, the same kind in your drinks. Chemically, it’s C2H5OH. Simple stuff.
2. Can I use sugarcane ethanol in my regular gasoline car?
Most gas cars can handle up to 10% ethanol (E10) without problems. If you want to use those super-high blends like E85, you’ll need a flex-fuel car. Otherwise, stick to the basics.
3. Does producing ethanol using sugarcane have a better environmental impact than corn-based ethanol?
Totally. Sugarcane gives you more ethanol per acre and needs less energy to grow and process. So yeah, it’s greener than corn.
4. Can sugarcane be used to produce ethanol and cause food shortages?
That’s a fair worry, but honestly, sugarcane doesn’t hog all the farmland. Smarter farming and better crops keep things in check. No one’s losing dinner over this.
5. What other uses are there for the byproducts produced from sugarcane ethanol?
Nothing goes to waste. They burn bagasse for power or use it for paper and building stuff. Vinasse? They use it as fertilizer. It’s all pretty efficient.







