The pharmaceutical and healthcare industry is changing fast. And making Manoeuvring through these changes in technology [(IV Fluid (BFS Technology)], IV fluids companies is looking very good indeed.
More hospitals are opening. Demand for sterile solutions injectable keeps going up, and safety regulations ever tighten. That there are these trends in the IV fluids market is a steady expansion.
If you’re a businessman who want to start BFS technology IV fluids production in 2025, This is your step by step introduction.
What are IV Fluids?
These are liquid solutions injected into a person’s circulation system (vein). They hydrate, providing electrolytes and nutrition, or thin down medication from the blood mix.
Doctors use them in operations emergencies situations, or whenever patients just can’t eat or drink–and usually need more fluids than normal due to lost blood.
What is Blow-Fill-Seal (BFS) Technology?
BFS (Blow-Fill-Seal) equipment is the latest automatic making sterile products. This machine turns plastic resins in the raw material to form a container, fills it with sterile fluid and then seals it–all in one process, within a closed-in environment.
The product has no exposure at all to human hands which reduces contamination risks and increases sterility. Also, it cuts down on labor costs and increases the output owing to efficiency thus making production more profitable still.
Why BFS Technology for IV Fluids?
Higher Safety Standards: Regulators are insisting on more sterility for injectibles. BFS tech satisfies these standards.
Growing Demand: Hospitals and medical centers need more IV fluids every year.
Efficient Production: Fully automated BFS machines allow companies to turn out their products quickly and with fewer people.
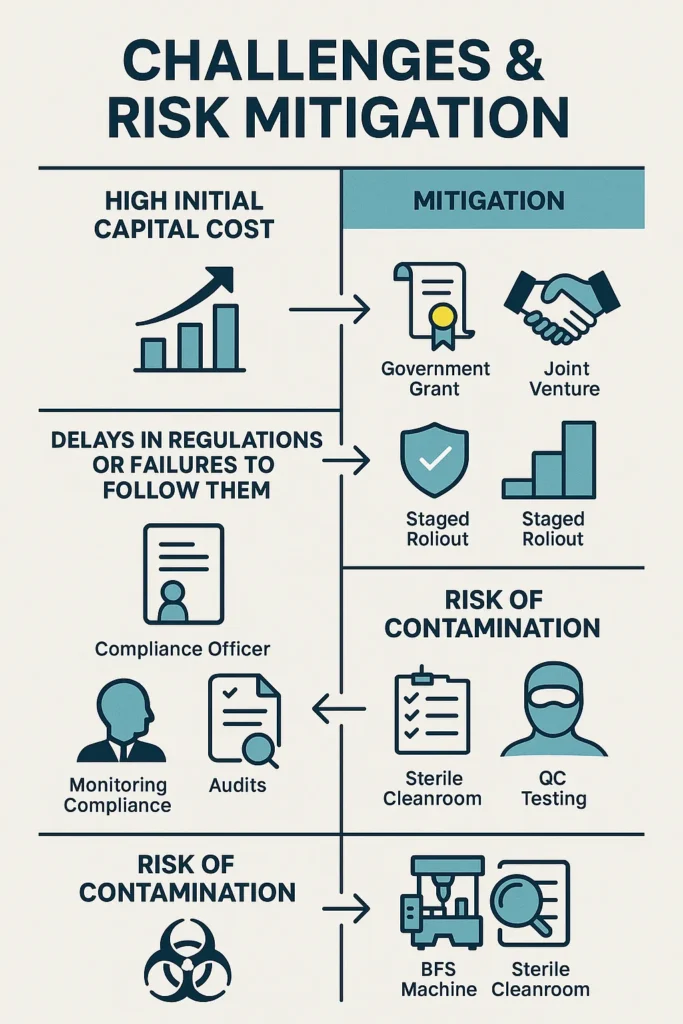
Challenges & Risk Mitigation
High Initial Capital Cost
- Look for government grants that are available.
- Join forces through joint ventures.
- Find safe ways to get money.
- To keep an eye on cash flow, roll out the project in stages.
Delays in regulations or failures to follow them
- Hire people who know a lot about regulations.
- Take care when making and reviewing documents.
- Do regular audits of your own work.
- Keep your quality systems strong.
Risk of contamination
- Use blow-fill-seal (BFS) technology to cut down on contact with people.
- Follow the rules for cleanrooms very carefully.
- Put in place full quality control measures.
How reliable is the supplier?
- Check out all of your suppliers very carefully.
- Get backup sources of supply.
- Make sure the quality of all raw materials by testing them.
Competition from well-known businesses
- Stand out by offering better quality and well-known certifications.
- Give people more choices of fluids and packaging.
- To stand out, you need to have a presence in your area or region.
Start an IV Fluids Business with BFS in Key Steps
Market Research & Demand Analysis
Research the local, national and international demand for IV fluids.
Hospitals, clinics, emergency providers and ambulance, nursing homes, home healthcare and veterinary services are all key presumptive customers.
Check on the incumbent suppliers, prices they offer, and whether they match your own development plans.
Investigate related policies and rules, such as those affecting imports and common guidelines for government procurement.
Read More: Production of I.V. Fluids (BFS Technology).
Product Line & Packaging Variants
Decide on the particular fluids you will offer: Normal Saline, various Dextrose Solutions, Ringer’s Lactate, Combination Fluids, Sterile Water for Injection
Choose the size of suitable packaging such as 100 milliliters, 250 ml, 500 ml and 1 liter
Between flexible bags or inflexible plastic containers choose based on local market demand.
Regulatory & Compliance Requirements
Gain necessary permission and permits through national drug regulatory authorities.
Comply with Good Manufacturing Practices (GMP) standards and, where relevant, WHO GMP standards.
Make sure that clean room standards are met and validated.
Get environmental, fire and safety approvals.
For batch tracking and quality control, establish a proper documentation system.
Exports are to be informed of the standards of various agencies, such as USFDA, EMA
Read Our Book: Click Here
Facility & Infrastructure Setup
Design your facility for the best flow of material.
That includes raw material storage, purified water systems, the preparation area, BFS and sealing, the QC lab, packaging and warehousing.
Away from contamination, well-constructed cleanrooms maintain proper air filtration and pressure conditions
Utilities that can be counted on: electricity, water, steam, air and waste management.
Read More: How to start the Manufacturing Unit of IV Fluid (BFS Technology)?
Machinery, Raw Materials & Technology
Key equipment to be purchased: BFS machinery, water purification and WFI systems, mixing tanks, sterile filtration units, leak detectors, and packaging machines.
Source polymers and pharmaceutical-grade chemicals from the industry (salts, Dextrose, electrolytes).
Needs to be satisfied include such things as sterile filters, seals and labels.
Staffing, Training & Quality Systems
For operations, quality control, regulatory matters, maintenance and services-need to hire knowledgeable people.
Preparation in procedures for development and documentation, business activity instructions, GMP discussion and in aseptic techniques
Be careful and have SOPs, resulting records and traceability
Read Our Project Report: Click Here
Marketing, Sales Channels & Distribution
Plan to approach both government hospitals and private ones by means of tenders and direct sales
Make connections with clinics, pharmacies, distributors and wholesalers
Explore export opportunities the quality of your product is up to international standards.
Concentrate on branding, dependability, certification and product quality.
Financial Planning: Investment, Costs & ROI
What’s the capital requirement for land, infrastructure, construction and machinery, so on?
Consider other expenses related to running utilities, raw material purchases, employment and required reporting.
A customary shortage period, generally speaking is 2-5 years based on the size of commericial operations in question and the contracts being fulfilled during that period.
To get started, you must be sure there are enough people willing and able to purchase IV fluids. Once that question has been answered definitively, any idea of moving ahead is worth considering.
Read Our Project Report: Click Here
Emerging Trends & Future Opportunities in 2025
Sustainable
In the future, demand for eco-friendly packaging will only go up. In order to reduce the amount of waste and greenhouse gas emissions they produce, businesses have been using recyclable and biodegradable plastics for their product materials.
Digital Monitoring & Automation
To ensure the reliability of their products, companies in agriculture have begun using IOT sensors and automatic data logging to measure and investigate things like stability, temperature, and humidity. The highest level of quality control is the form that takes place in real time.
Personalized Medicines and IV Fluids Niche
Specialized intravenous fluids are in great demand. This includes formulations which can be used for nutritional therapy, pediatrics and even veterinary purposes.
Resilience of Supply Chain & Local Manufacturing
Many areas are now focused on self-sufficiency. Through local manufacturing entrepreneurship, supply chains have meanwhile been building to increase resilience and reduce reliance on imports.
More stringent regulation
Authorities are imposing stricter GMP and WHO standards. Companies must endure more stringent verification procedures and endure increased import/export checks.
Find the Best Idea for Yourself With our Startup Selector Tool
Conclusion
2025 appears to be a good year to start using BFS technology in IV. With rising industry demand and strong regulatory standards shaping the market, it represents a solid opportunity.
IV Fluid (BFS Technology): Five common questions from the FAQ
Q1: How much investment is typically needed to start a business manufacturing IV fluids by BFS technology?
The investment needed varies by the size of the plant, the level of automation in place, the location, how old the building is and what condition it is in, and the requirements for a separate controlled environment area.
As an approximation, for a mid-sized facility, costs will run into crores of rupees or millions of US dollars. Please remember as well operational and working capital costs.
Q2: Which regulatory approvals are essential?
In addition to a drug manufacturing license, producers must achieve one or other of the key international GMP (Good Manufacturing Practice) certifications.
They also need environmental clearance, cleanroom certifications, laboratory efficacy validation and documented quality assurance. Exporters must also meet FDA or EMA standards.
Q3: Which types of IV fluids are most in demand?
Core products include normal saline (0.9% sodium chloride), dextrose solutions (usually 5%), Ringer’s Lactate, combination fluids (e.g., dextrose with saline) and sterile water for injections. Specialty and niche fluids are also in increasing demand.
Q4: How should I start this?
To begin with 100ml, 250ml, 500ml and 1L pack sizes. We prefer using plastic BFS ( Blow-Fill-Seal ) containers due to safety and convenience. Always check regulatory approval for your packaging materials in your target market.
Q5: What about breakeven timeline and profitability outlook?
If operation is efficient and there is strong demand, it commonly takes 2-5 years to break even. Profitability depends on your scale of production, quality control, material costs and overall efficiency. Automation and robust quality assurance systems will help margins improve.