Saline solution is a life-saving medical commodity around the world, but few people understand how complex and vital its production process can be.
That in every back of saline is an elaborate process of manufacturing the solution that draws from strict global patterns, sophisticated technology and robust supply chains.
In 2025, it was never more critical to produce saline. And the use of sterile saline solutions has countless applications, from helping to protect millions of surgeries each year to serving as an emergency front line product.
This NPCS blog will tell you everything you must know regarding it: global market standpoint, opportunities and challenges, and what future holds.
Read Our Book: Click Here
What is Saline Manufacturing
The manufacturing of saline is the preparation of sterile salt water solutions, usually at 0.9% concentration in purified water.
This isotonic solution is used for:
- Intravenous Hydration (IV)
- Diluting medicines
- Wound irrigation
- Medical procedures such as dialysis
Even though it may seem so simple, the medical grade saline has to be held to a high standard of safety and quality. The slightest contamination or concentration mistake can literally kill a patient, so saline ain’t that at the easy street in terms of regulations.
Read More: Biodegradable Products Business: Profitable Eco-Friendly Opportunities in 2025
Regulatory and quality standards
Saline solution production is not just to mix salt and water. Each lot should comply with good international manufacturing (GMP) practices and undergo strict tests.
- Water Quality: It must be water for injection (WFI) or equivalent. Sterility: Solutions are sterilized by autoclave or sterile filtration.
- Tests: The solution is tested for pH, presence of particles, pyrogenns and endotoxins.
- Packaging: Plastics or medical level glass should be validated to avoid leaching.
Regulatory supervision comes from: FDA (USA), EMA (Europe), CDSCO (India), WHO guidelines for export.
Challenges in Saline Manufacture
Even though it is a vital product, saline solution producers have major challenges:
Supply chain vulnerabilities: If packaging runs low, you’ve got a global scarcity, or raw salt, or if installation shuts down.
- Slim margins: Saline is a low-margin product, so profits are based on efficiencies.
- Regulatory barriers: Lots of inspections and certifications add cost.
- Contamination risks: Tiny breaks in sterility may result in recalls.
In fact, the F.D.A. recently declared that shortages of saline had finally ended in mid-2025, following years when supply was limited by hurricanes that damaged repair-deprived main United States plants.
Read Our Project Report: Click Here
Industry SWOT Analysis
- Strengths: The manufacture of saline benefits from constant demand in health care and a simple and highly scalable formula, making it a reliable product in global markets.
- Weaknesses: The sector faces high compliance costs due to rigid regulations and usually operates with fine profit margins.
- Opportunities: There is a strong growth potential in Asia-Pacific, along with innovations in balanced saline packaging and solutions.
- Threats: The main risks include the scarcity of disaster supplies, as well as intense pressures and competition prices.
The Saline Manufacturing Process
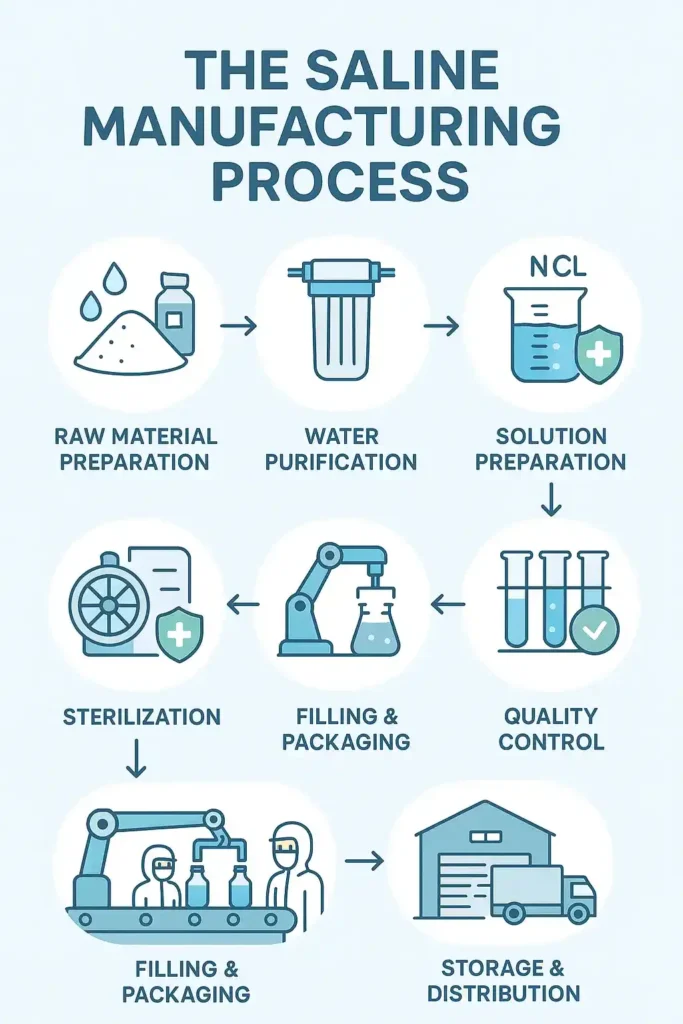
Raw Material Preparation: This involves the use of pharmaceutical quality salt and distilled water. The contents of the sterile bags, bottles and syringes are checked prior to use.
Water Purification: Water is purified through reverse osmosis, distillation or with the use of UV. All that does not pass testing goes on into the processes.
Solution Preparation: 100 g of sodium chloride was weighed accurately and dissolved in purified water homogenized with mixing. Automated systems ensure accuracy.
Sterilization: Sterilization is performed by autoclaves or sterile filtration. Repeat testing demonstrates the destruction of dangerous microorganisms.
Filling & Packaging: Robotic machines fill and seal containers in clean rooms. All workers don protective suits to keep everything sterile.
Quality Control: Every batch is subject to sterility, pH and concentration testing. Only batches that pass are released for distribution.
Storage & Distribution: Products are kept in temperature controlled environments. The shipments are monitored, and any damaged deliveries are refused on the spot.
Read More: Complete Guide to Building and Scaling a Manufacturing Business
How to Start a Saline Manufacturing Business
Market Research: Check what the hospitals want, which rule the market, and which licensing hoops jump through you.
Close regulations: You cannot ignore paperwork. Apply for FDA, GMP, or CDSCO permit based on your area.
Facility Setup: Invest cash in legal goods- Industrial-grade water purifiers, sterile filling machines and bulletproof packaging lines. The key to sterility, so do not make it cheaper here.
A-team construction: You want legitimate microbiologist, sharp engineer, plus a passionate quality control squad. This is not kitchen science, so skilled experts are your tickets for constant quality.
Secure the reliable supply chains: Close reliable sources for raw salt and packaging rapid packaging. Smooth supply means that you prevent production anytime. Create concrete relations with distributors to avoid the supply chain surprise.
Choosing sales channel: Target your efforts on hospitals, clinics and large times wholesalers of time. Do not ignore government tenders – if you play your card correctly they can turn into fat recurrence contracts.
Read More: Pharmaceutical Manufacturing Trends for 2025: Shaping the Future
Future prospects
The saltwater industry in 2025 is positioned for steady, long -term growth. Global demand will continue to increase, especially in emerging economies. With several governments that emphasize domestic production capacity, new players will enter the market.
Expect to see:
- Widespread adoption of balanced crystalloids
- Packaging innovations aimed at sustainability
- Digitization of production facilities for better traceability
- Stronger regulations on safety and environmental footprint
Find the Best Idea for Yourself With our Startup Selector Tool
Conclusion
The saline may seem simple -only salt and water – but producing it plays a critical role in global health care. While we look at 2025 and beyond, the saline will continue to expand, fuelled by innovation, growing demand and governments that press through resilient health supply chains.
For investors, entrepreneurs and health professionals, understanding the nuances of saline manufacturing is not just about business – it is about contributing to one of the most essential pillars of modern medicine. For more assistance to launch you business in this industry, contact us.
Saline Manufacturing: Frequently Asked Questions
Q1. Why is the saline solution always 0.9% sodium chloride?
Because it is isotonic with human blood plasma, which means it does not cause harmful fluid changes inside or outside cells.
Q2. What caused a recent saline scarcity?
Natural disasters impairing factories, scarcity of stories -unexpected prices and peaks in demand.
Q3. Is it profitable to start a saline factory?
Yes, but only on scale. The product has low margins, therefore, profitability depends on efficiency, automation and long -term contracts.
Q4. What regulations govern saline manufacturing?
Good manufacturing practices (GMP), FDA (USA), EMA (Europe), CDSCO (India) and WHO guidelines for global exports.
Q5. What are the latest trends in the manufacture of saline?
Balanced salt solutions, local production initiatives, ecological packaging and automated quality control systems.